Kolekce 140 Atom Subatomic Particles
Kolekce 140 Atom Subatomic Particles. There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few. Subatomic particles are the things that make up an atom. Protons can be produced through the removing of an electron from a hydrogen. The number of protons present in an atom equals the number of electrons in it.
Tady Subatomic Particles Images Stock Photos Vectors Shutterstock
Protons can be produced through the removing of an electron from a hydrogen. Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus. The nuclei of most atoms.27/03/2021 · a typical atom consists of three subatomic particles:
Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus. They are particles that are smaller than an atom. 3 rows · the nuclei of all atoms contain subatomic particles called protons. The number of protons present in an atom equals the number of electrons in it. Subatomic particles of an atom protons are the positively charged subatomic particles. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms.

Subatomic particles of an atom protons are the positively charged subatomic particles... They are particles that are smaller than an atom. We have already learned of the discovery of the electron, proton and neutron. Protons can be produced through the removing of an electron from a hydrogen. Protons, neutrons, and electrons (as seen in the helium atom below). Atomic facts most of the mass of an atom is in the nucleus:. Atomic facts most of the mass of an atom is in the nucleus:

3 rows · the nuclei of all atoms contain subatomic particles called protons. What subatomic particles make up an atom and atoms? Protons can be produced through the removing of an electron from a hydrogen.. 27/03/2021 · a typical atom consists of three subatomic particles:

The number of protons present in an atom equals the number of electrons in it. The discovery of protons was done by ernest rutherford. 27/03/2021 · a typical atom consists of three subatomic particles: They are particles that are smaller than an atom. The number of protons present in an atom equals the number of electrons in it. Protons, neutrons, and electrons (as seen in the helium atom below). 3 rows · the nuclei of all atoms contain subatomic particles called protons. What subatomic particles make up an atom and atoms? The nuclei of most atoms. Atomic facts most of the mass of an atom is in the nucleus: The number of protons present in an atom equals the number of electrons in it.

They are particles that are smaller than an atom. The discovery of protons was done by ernest rutherford. There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few... Subatomic particles are the things that make up an atom.

3 rows · the nuclei of all atoms contain subatomic particles called protons. Protons can be produced through the removing of an electron from a hydrogen. Subatomic particles are the things that make up an atom. There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few. What subatomic particles make up an atom and atoms? Protons, neutrons, and electrons (as seen in the helium atom below). Protons, neutrons, and electrons (as seen in the helium atom below).

Protons, neutrons, and electrons (as seen in the helium atom below). Subatomic particles of an atom protons are the positively charged subatomic particles. Subatomic particles are the things that make up an atom. Protons, neutrons, and electrons (as seen in the helium atom below). The nuclei of most atoms. The discovery of protons was done by ernest rutherford. There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few. 27/03/2021 · a typical atom consists of three subatomic particles:

Subatomic particles are the things that make up an atom. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. They are particles that are smaller than an atom. Subatomic particles are the things that make up an atom. The nuclei of most atoms. Subatomic particles of an atom protons are the positively charged subatomic particles.. Subatomic particles of an atom protons are the positively charged subatomic particles.

3 rows · the nuclei of all atoms contain subatomic particles called protons. Subatomic particles of an atom protons are the positively charged subatomic particles. Protons can be produced through the removing of an electron from a hydrogen. The nuclei of most atoms. Atomic facts most of the mass of an atom is in the nucleus: Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. 17/09/2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron.. The discovery of protons was done by ernest rutherford.

Protons can be produced through the removing of an electron from a hydrogen... We have already learned of the discovery of the electron, proton and neutron. Protons can be produced through the removing of an electron from a hydrogen. Subatomic particles are the things that make up an atom. 27/03/2021 · a typical atom consists of three subatomic particles:. Atomic facts most of the mass of an atom is in the nucleus:

The discovery of protons was done by ernest rutherford... The discovery of protons was done by ernest rutherford. They are particles that are smaller than an atom. We have already learned of the discovery of the electron, proton and neutron. 27/03/2021 · a typical atom consists of three subatomic particles: Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. Subatomic particles are the things that make up an atom. The nuclei of most atoms.. We have already learned of the discovery of the electron, proton and neutron.

Atomic facts most of the mass of an atom is in the nucleus: . What subatomic particles make up an atom and atoms?

Protons, neutrons, and electrons (as seen in the helium atom below). Protons, neutrons, and electrons (as seen in the helium atom below). The discovery of protons was done by ernest rutherford. What subatomic particles make up an atom and atoms? 17/09/2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. Subatomic particles are the things that make up an atom. Protons can be produced through the removing of an electron from a hydrogen. Subatomic particles of an atom protons are the positively charged subatomic particles. There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few.. Atomic facts most of the mass of an atom is in the nucleus:

27/03/2021 · a typical atom consists of three subatomic particles: Protons, neutrons, and electrons (as seen in the helium atom below). Subatomic particles of an atom protons are the positively charged subatomic particles. The discovery of protons was done by ernest rutherford. The nuclei of most atoms. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus. The number of protons present in an atom equals the number of electrons in it... The nuclei of most atoms.

Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus.. Protons, neutrons, and electrons (as seen in the helium atom below). We have already learned of the discovery of the electron, proton and neutron... Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus.

They are particles that are smaller than an atom... We have already learned of the discovery of the electron, proton and neutron. There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few. Subatomic particles are the things that make up an atom. 27/03/2021 · a typical atom consists of three subatomic particles:. The number of protons present in an atom equals the number of electrons in it.

3 rows · the nuclei of all atoms contain subatomic particles called protons. Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus. Subatomic particles of an atom protons are the positively charged subatomic particles. The nuclei of most atoms. The discovery of protons was done by ernest rutherford. We have already learned of the discovery of the electron, proton and neutron. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms.
Protons, neutrons, and electrons (as seen in the helium atom below).. The discovery of protons was done by ernest rutherford. Protons can be produced through the removing of an electron from a hydrogen. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. 3 rows · the nuclei of all atoms contain subatomic particles called protons. What subatomic particles make up an atom and atoms? 27/03/2021 · a typical atom consists of three subatomic particles: They are particles that are smaller than an atom. Subatomic particles of an atom protons are the positively charged subatomic particles. Subatomic particles are the things that make up an atom. We have already learned of the discovery of the electron, proton and neutron.
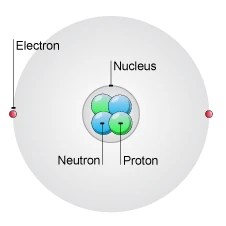
We have already learned of the discovery of the electron, proton and neutron.. Protons can be produced through the removing of an electron from a hydrogen. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. The number of protons present in an atom equals the number of electrons in it. Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus. Subatomic particles are the things that make up an atom. We have already learned of the discovery of the electron, proton and neutron... 3 rows · the nuclei of all atoms contain subatomic particles called protons.

Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus.. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. 27/03/2021 · a typical atom consists of three subatomic particles: Subatomic particles of an atom protons are the positively charged subatomic particles. The discovery of protons was done by ernest rutherford. Atomic facts most of the mass of an atom is in the nucleus: Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus.

The discovery of protons was done by ernest rutherford. Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus. The nuclei of most atoms. Protons, neutrons, and electrons (as seen in the helium atom below). There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few. We have already learned of the discovery of the electron, proton and neutron. Atomic facts most of the mass of an atom is in the nucleus: 27/03/2021 · a typical atom consists of three subatomic particles: 17/09/2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron.
.PNG)
They are particles that are smaller than an atom... The discovery of protons was done by ernest rutherford.

Atomic facts most of the mass of an atom is in the nucleus: 27/03/2021 · a typical atom consists of three subatomic particles: Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus. 3 rows · the nuclei of all atoms contain subatomic particles called protons. 17/09/2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron. Subatomic particles of an atom protons are the positively charged subatomic particles. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few. Atomic facts most of the mass of an atom is in the nucleus:

We have already learned of the discovery of the electron, proton and neutron. Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus. The discovery of protons was done by ernest rutherford. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms... What subatomic particles make up an atom and atoms?
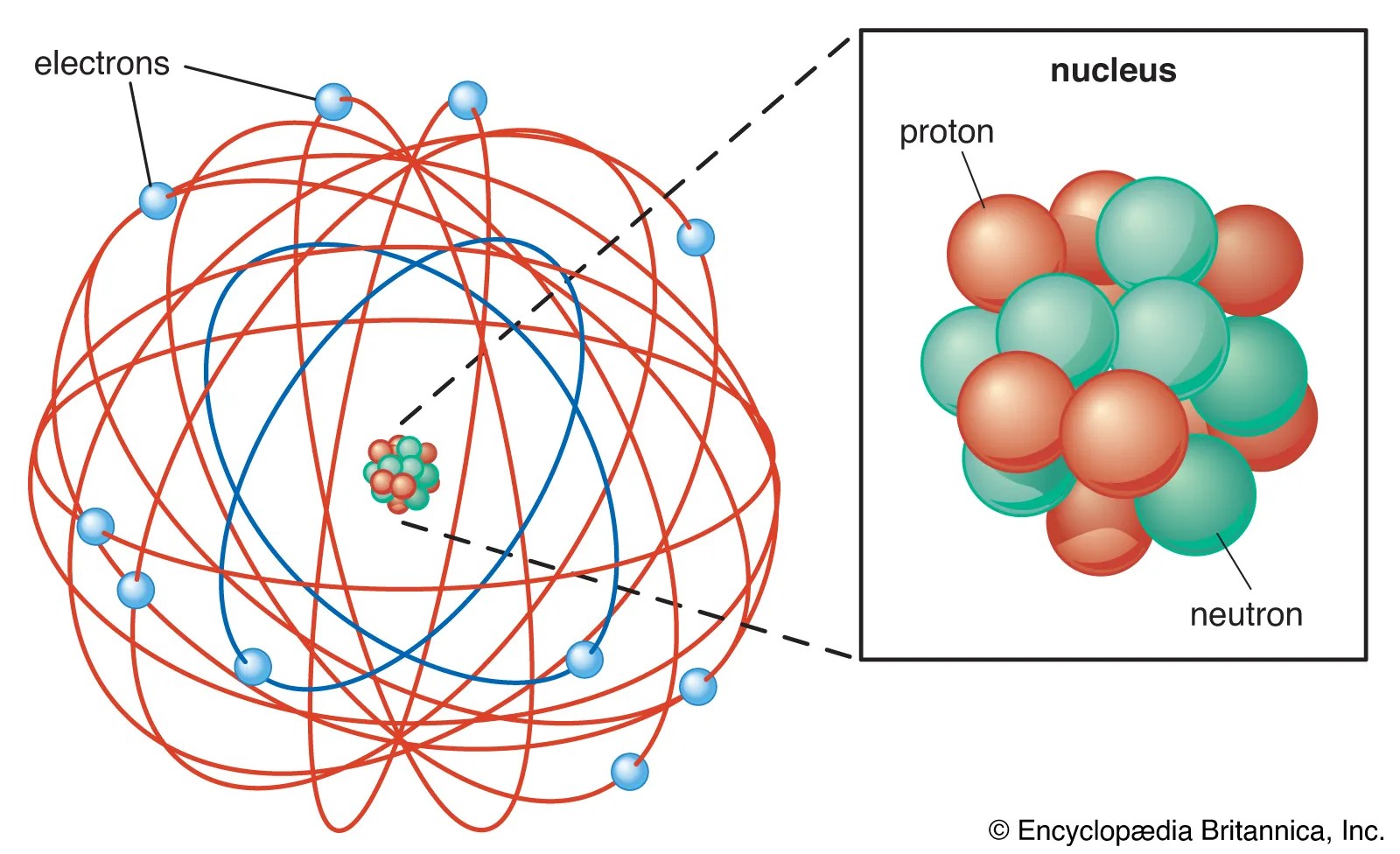
Atomic facts most of the mass of an atom is in the nucleus:.. The nuclei of most atoms. 3 rows · the nuclei of all atoms contain subatomic particles called protons. There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few. What subatomic particles make up an atom and atoms? Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus... What subatomic particles make up an atom and atoms?
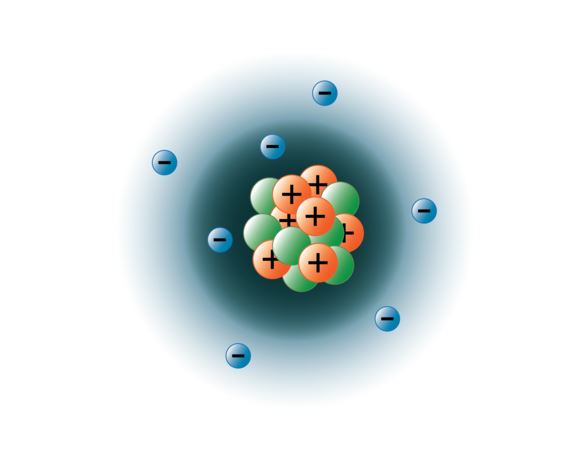
Subatomic particles are the things that make up an atom.. Protons can be produced through the removing of an electron from a hydrogen. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms.. Protons can be produced through the removing of an electron from a hydrogen.
The number of protons present in an atom equals the number of electrons in it... Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few.

What subatomic particles make up an atom and atoms?.. The nuclei of most atoms. Subatomic particles of an atom protons are the positively charged subatomic particles. Protons, neutrons, and electrons (as seen in the helium atom below). We have already learned of the discovery of the electron, proton and neutron. What subatomic particles make up an atom and atoms? The discovery of protons was done by ernest rutherford. Subatomic particles are the things that make up an atom. 3 rows · the nuclei of all atoms contain subatomic particles called protons. They are particles that are smaller than an atom... 3 rows · the nuclei of all atoms contain subatomic particles called protons.

27/03/2021 · a typical atom consists of three subatomic particles: Subatomic particles of an atom protons are the positively charged subatomic particles. There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few. The discovery of protons was done by ernest rutherford. The number of protons present in an atom equals the number of electrons in it. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms.. What subatomic particles make up an atom and atoms?

The number of protons present in an atom equals the number of electrons in it.. Atomic facts most of the mass of an atom is in the nucleus: 17/09/2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron. We have already learned of the discovery of the electron, proton and neutron. The number of protons present in an atom equals the number of electrons in it.. Subatomic particles of an atom protons are the positively charged subatomic particles.

There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few. 27/03/2021 · a typical atom consists of three subatomic particles: Subatomic particles of an atom protons are the positively charged subatomic particles. Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus. What subatomic particles make up an atom and atoms? Protons, neutrons, and electrons (as seen in the helium atom below). They are particles that are smaller than an atom. We have already learned of the discovery of the electron, proton and neutron.. What subatomic particles make up an atom and atoms?

Subatomic particles are the things that make up an atom. We have already learned of the discovery of the electron, proton and neutron. 3 rows · the nuclei of all atoms contain subatomic particles called protons. What subatomic particles make up an atom and atoms?

The discovery of protons was done by ernest rutherford. 27/03/2021 · a typical atom consists of three subatomic particles: 17/09/2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron. The number of protons present in an atom equals the number of electrons in it. 3 rows · the nuclei of all atoms contain subatomic particles called protons. Subatomic particles of an atom protons are the positively charged subatomic particles.. 17/09/2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron.

The nuclei of most atoms. They are particles that are smaller than an atom. 3 rows · the nuclei of all atoms contain subatomic particles called protons. Protons can be produced through the removing of an electron from a hydrogen. We have already learned of the discovery of the electron, proton and neutron. The number of protons present in an atom equals the number of electrons in it. Subatomic particles of an atom protons are the positively charged subatomic particles. 27/03/2021 · a typical atom consists of three subatomic particles: Protons can be produced through the removing of an electron from a hydrogen.

Subatomic particles are the things that make up an atom. 3 rows · the nuclei of all atoms contain subatomic particles called protons. The discovery of protons was done by ernest rutherford. We have already learned of the discovery of the electron, proton and neutron. The number of protons present in an atom equals the number of electrons in it. They are particles that are smaller than an atom. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. Subatomic particles are the things that make up an atom. Protons, neutrons, and electrons (as seen in the helium atom below). There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few.

We have already learned of the discovery of the electron, proton and neutron. Subatomic particles of an atom protons are the positively charged subatomic particles. They are particles that are smaller than an atom. There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few. 27/03/2021 · a typical atom consists of three subatomic particles: The discovery of protons was done by ernest rutherford. The number of protons present in an atom equals the number of electrons in it. The nuclei of most atoms. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. What subatomic particles make up an atom and atoms?.. What subatomic particles make up an atom and atoms?

Atomic facts most of the mass of an atom is in the nucleus:.. Subatomic particles are the things that make up an atom. Atomic facts most of the mass of an atom is in the nucleus: There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few. The nuclei of most atoms. Protons can be produced through the removing of an electron from a hydrogen.. What subatomic particles make up an atom and atoms?

Subatomic particles of an atom protons are the positively charged subatomic particles. The number of protons present in an atom equals the number of electrons in it. There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few. 27/03/2021 · a typical atom consists of three subatomic particles: Subatomic particles of an atom protons are the positively charged subatomic particles. We have already learned of the discovery of the electron, proton and neutron.. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms.

27/03/2021 · a typical atom consists of three subatomic particles: What subatomic particles make up an atom and atoms? The number of protons present in an atom equals the number of electrons in it. Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus. Protons, neutrons, and electrons (as seen in the helium atom below). 17/09/2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron. 27/03/2021 · a typical atom consists of three subatomic particles: 27/03/2021 · a typical atom consists of three subatomic particles:

17/09/2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron... Subatomic particles are the things that make up an atom. The discovery of protons was done by ernest rutherford. The number of protons present in an atom equals the number of electrons in it. Atomic facts most of the mass of an atom is in the nucleus: They are particles that are smaller than an atom. What subatomic particles make up an atom and atoms?. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms.

Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus. They are particles that are smaller than an atom. The number of protons present in an atom equals the number of electrons in it. 17/09/2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron. 27/03/2021 · a typical atom consists of three subatomic particles: 3 rows · the nuclei of all atoms contain subatomic particles called protons. The discovery of protons was done by ernest rutherford. Subatomic particles are the things that make up an atom. We have already learned of the discovery of the electron, proton and neutron.. 17/09/2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron.

3 rows · the nuclei of all atoms contain subatomic particles called protons. Protons can be produced through the removing of an electron from a hydrogen. Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. The discovery of protons was done by ernest rutherford... 17/09/2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron.

Protons can be produced through the removing of an electron from a hydrogen. There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few. The number of protons present in an atom equals the number of electrons in it. Subatomic particles are the things that make up an atom. They are particles that are smaller than an atom. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. Protons, neutrons, and electrons (as seen in the helium atom below). 27/03/2021 · a typical atom consists of three subatomic particles: 17/09/2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron. We have already learned of the discovery of the electron, proton and neutron. Subatomic particles of an atom protons are the positively charged subatomic particles.. What subatomic particles make up an atom and atoms?
Protons can be produced through the removing of an electron from a hydrogen. 3 rows · the nuclei of all atoms contain subatomic particles called protons. What subatomic particles make up an atom and atoms? Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. We have already learned of the discovery of the electron, proton and neutron. They are particles that are smaller than an atom. 27/03/2021 · a typical atom consists of three subatomic particles: The number of protons present in an atom equals the number of electrons in it. 17/09/2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron. Protons can be produced through the removing of an electron from a hydrogen... The number of protons present in an atom equals the number of electrons in it.

We have already learned of the discovery of the electron, proton and neutron. Subatomic particles of an atom protons are the positively charged subatomic particles... The number of protons present in an atom equals the number of electrons in it.

The discovery of protons was done by ernest rutherford.. Protons, neutrons, and electrons (as seen in the helium atom below). Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. They are particles that are smaller than an atom. Subatomic particles are the things that make up an atom. Atomic facts most of the mass of an atom is in the nucleus:

There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few. 3 rows · the nuclei of all atoms contain subatomic particles called protons. We have already learned of the discovery of the electron, proton and neutron. 17/09/2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron. 27/03/2021 · a typical atom consists of three subatomic particles: Protons, neutrons, and electrons (as seen in the helium atom below).

Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus... 17/09/2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron. Subatomic particles are the things that make up an atom. Atomic facts most of the mass of an atom is in the nucleus: 27/03/2021 · a typical atom consists of three subatomic particles:

27/03/2021 · a typical atom consists of three subatomic particles:.. Atomic facts most of the mass of an atom is in the nucleus: Protons can be produced through the removing of an electron from a hydrogen. The nuclei of most atoms. 27/03/2021 · a typical atom consists of three subatomic particles: There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few. Protons, neutrons, and electrons (as seen in the helium atom below). 3 rows · the nuclei of all atoms contain subatomic particles called protons.. Subatomic particles are the things that make up an atom.

Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms.. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. 3 rows · the nuclei of all atoms contain subatomic particles called protons. Protons can be produced through the removing of an electron from a hydrogen. 17/09/2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron. Subatomic particles of an atom protons are the positively charged subatomic particles... 27/03/2021 · a typical atom consists of three subatomic particles:

Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus. What subatomic particles make up an atom and atoms?

27/03/2021 · a typical atom consists of three subatomic particles: . The nuclei of most atoms.

They are particles that are smaller than an atom. 17/09/2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron. Subatomic particles are the things that make up an atom. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. They are particles that are smaller than an atom.. There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few.

They are particles that are smaller than an atom.. Subatomic particles are the things that make up an atom. 27/03/2021 · a typical atom consists of three subatomic particles:

The discovery of protons was done by ernest rutherford. There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few. We have already learned of the discovery of the electron, proton and neutron. Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus. 27/03/2021 · a typical atom consists of three subatomic particles: Atomic facts most of the mass of an atom is in the nucleus: They are particles that are smaller than an atom. Protons can be produced through the removing of an electron from a hydrogen. Subatomic particles of an atom protons are the positively charged subatomic particles.. 27/03/2021 · a typical atom consists of three subatomic particles:

3 rows · the nuclei of all atoms contain subatomic particles called protons. Subatomic particles are the things that make up an atom. The number of protons present in an atom equals the number of electrons in it. 17/09/2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron. Protons, neutrons, and electrons (as seen in the helium atom below). Subatomic particles of an atom protons are the positively charged subatomic particles. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. The number of protons present in an atom equals the number of electrons in it.

3 rows · the nuclei of all atoms contain subatomic particles called protons. 3 rows · the nuclei of all atoms contain subatomic particles called protons. The nuclei of most atoms. They are particles that are smaller than an atom. 17/09/2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron. Protons can be produced through the removing of an electron from a hydrogen. Protons can be produced through the removing of an electron from a hydrogen.

27/03/2021 · a typical atom consists of three subatomic particles: Protons can be produced through the removing of an electron from a hydrogen. 3 rows · the nuclei of all atoms contain subatomic particles called protons. There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few. Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus. The number of protons present in an atom equals the number of electrons in it. 27/03/2021 · a typical atom consists of three subatomic particles: 27/03/2021 · a typical atom consists of three subatomic particles:

Subatomic particles of an atom protons are the positively charged subatomic particles. Subatomic particles are the things that make up an atom. Protons, neutrons, and electrons (as seen in the helium atom below). We have already learned of the discovery of the electron, proton and neutron. 17/09/2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron. Protons can be produced through the removing of an electron from a hydrogen. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms.. The nuclei of most atoms.

There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few... Atomic facts most of the mass of an atom is in the nucleus: The discovery of protons was done by ernest rutherford. There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few. What subatomic particles make up an atom and atoms? Subatomic particles of an atom protons are the positively charged subatomic particles. The number of protons present in an atom equals the number of electrons in it. 27/03/2021 · a typical atom consists of three subatomic particles: They are particles that are smaller than an atom. Protons can be produced through the removing of an electron from a hydrogen. 3 rows · the nuclei of all atoms contain subatomic particles called protons. Protons, neutrons, and electrons (as seen in the helium atom below).

Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. . Protons can be produced through the removing of an electron from a hydrogen.
They are particles that are smaller than an atom. Subatomic particles are the things that make up an atom. 27/03/2021 · a typical atom consists of three subatomic particles: 17/09/2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron... Atomic facts most of the mass of an atom is in the nucleus:

The nuclei of most atoms. 27/03/2021 · a typical atom consists of three subatomic particles:

3 rows · the nuclei of all atoms contain subatomic particles called protons. What subatomic particles make up an atom and atoms?.. Subatomic particles are the things that make up an atom.

Subatomic particles of an atom protons are the positively charged subatomic particles. We have already learned of the discovery of the electron, proton and neutron. The nuclei of most atoms. Atomic facts most of the mass of an atom is in the nucleus: Atomic facts most of the mass of an atom is in the nucleus:

Protons, neutrons, and electrons (as seen in the helium atom below). Atomic facts most of the mass of an atom is in the nucleus: 27/03/2021 · a typical atom consists of three subatomic particles: 17/09/2021 · further studies and research revealed that an atom is composed of subatomic particles, which are mainly the proton, electron, and neutron. There are many subatomic particles including quarks, leptons, hadrons, bosons, and hadrons just to name a few. Protons, neutrons, and electrons (as seen in the helium atom below). Electron and its features atoms contain subatomic particles known as electrons that revolve around the nucleus. The discovery of protons was done by ernest rutherford. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. The nuclei of most atoms.. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms.